Thermodynamic systems : In the sense of study thermodynamics ,The universe is divided into two parts, one is the system, and the other is surrounding. The part of the universe that is considered or under observation is called the “system” and all that is in the universe except system is called the “surrounding”. It is the region in which estimates are made in relation to the system.
For example, if you are boiling something in cooker in your kitchen, then the cooker is the system and all other things except cooker in the kitchen is called surrounding.
What is a thermodynamic system?
Before learning Thermodynamic systems : Open, Closed and Isolated system, you must have to know about what is exactly a thermodynamic system?
A thermodynamic system is a finite area under which any thermodynamic process takes place.
“A thermodynamic system is the body of an object and / or radiation, enclosed in a space by walls, with defined features, which separate it from its surroundings.” The surroundings may include other thermodynamic systems, or portable systems that are not thermodynamic systems.
The wall of a thermodynamic system may be merely a theory, in which it is described as ‘permeable’ to all objects, all rays, and all energies. The state of the thermodynamic system can be fully described in several different ways, with several different sets of thermodynamic state variables.
Types of thermodynamic systems
The thermodynamic systems mainly divided in 3 parts. All three are explained below with example.
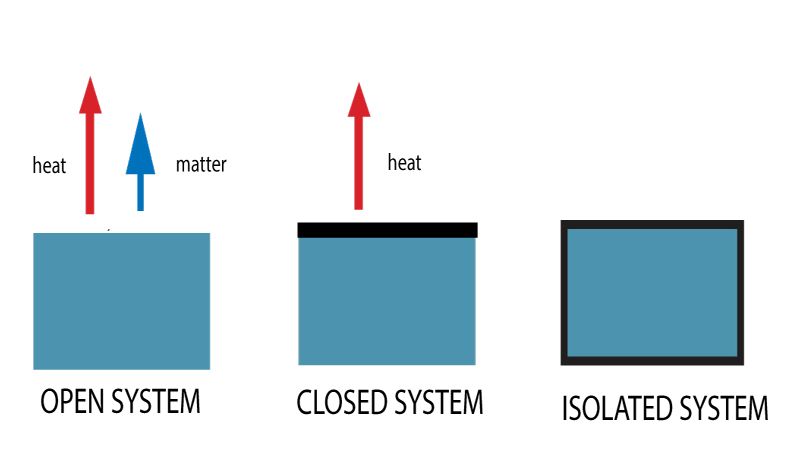
- Open system
A system that can transfer or exchange both mass and energy with its surrounding is called an open system. They are part and parcel of a large system. Open systems are sometimes called flow systems, due to the ability to exchange mass of the substance.
Examples of open system
• The human body.
• Automobile/ Car engine.
• Water boiling vessel, where water can evaporate and the vessel does not protect the inside at all.
- Closed system
A system that can only transfer or exchange the energy not mass with its surrounding is called a closed system. The mass cannot be transferred in a closed system.
Closed systems are less likely to be part of larger systems and are not fully connected to them. Closed systems are sometimes called non – flow processes, due to the inability to exchange mass, over a specific process.
Examples of closed system
• The earth is a closed system. It gets a lot of energy from the sun but the exchange of mass outside does not happen.
• Covered boiling water. It is a closed system because only heat energy can flow in or out, the amount of material (water) will remain the same unless it is disturbed.
• Putting an ice bag on the wound with a special bag or plastic to keep water out, if there is a wound.
- Isolated system
A system in which neither energy nor mass can transfer or exchange with the surrounding is called an isolated system. A isolated system may be part of a larger system, but it does not communicate with outsiders in any way.
Examples of an isolated system
• Burning glucose in a bomb calorimeter is an isolated system unless other real-life situations occur.
• A closed thermos bottle or a closed vacuum flask is actually an isolated system when the real difference is avoided.
Thermodynamic systems – So, that all from today’s article. You got to know about Types of thermodynamic systems. Also the definition and example of open, close and isolated system. Hope you all like this information.
If this was helpful to you then kindly share this with your friends.